

Information
What is radiation?
Radiation is a process by which energetic particles or energetic waves travel through a vacuum or through a medium containing no matter necessary for their propagation. Two energies of radiation are generally distinguished by the way they interact with common chemicals: ionizing and non-ionizing radiation. The word radiation is often used to refer to ionizing radiation (ie, radiation with enough energy to ionize an atom), but the term radiation can also refer to non-ionizing radiation (eg, radio waves, heat, or visible light). Particles or waves radiate from a source (ie, travel outward in all directions). Particles or waves radiate from a source (ie, travel outward in all directions). This aspect leads to a system of measurements and physical units applicable to all types of radiation. Because radiation travels through space and its energy is conserved in a vacuum, the strength of all forms of radiation follows an inverse relationship with distance from its source.
Both ionizing and non-ionizing radiation can harm living organisms and cause changes in the natural environment.
However, in general, ionizing radiation is more harmful to living organisms per unit of energy invested than non-ionizing radiation, because the ions produced by ionizing radiation, even at low radiation power, have the ability to cause DNA damage. In contrast, most non-ionizing radiations are harmful to living organisms only in proportion to their thermal energy, and have traditionally been considered harmless at low intensities that do not cause significant increases in temperature. In some aspects, ultraviolet radiation comprises a medium having certain characteristics of both ionizing and non-ionizing radiation. Although almost all of the UV spectrum of radiation is non-ionizing, at the same time UV radiation causes more damage to many molecules in biological systems than is accounted for by heating effects (sunburn is an example). These properties derive from UV's power to change chemical bonds, even though it does not have enough energy to ionize atoms.

Information
What radiation levels are considered safe?
The first step is to determine what your estimated annual radiation dose level is. There are many sources of radiation, some natural and some man-made.
Cosmic Radiation
This is radiation from space, from the Sun and other stars. It is partially blocked by Earth's atmosphere, so the higher you are, the less air there is to block it and the higher the level. It varies from 25 millirem (MR) per year at sea level to 50 millirem (MR) per year at an altitude of 1 mile. At two miles, this would be about 100 millirems (mR) a year.
This is the reason why you get a small amount of radiation while flying. Airplanes fly at altitudes of several miles, and fortunately the duration of a flight is only a few hours. A typical dose rate is 0.5 millirem (mR) per hour of flight.
Terrestrial Radiation
This is due to radiation from uranium, thorium and other radioactive materials found naturally in the soil. The average value is about 30 millirems (mR) per year, although it is much lower in coastal areas, about half.
Inhaled Radon is estimated at around 200 millirem (mR) a year
Other Sources of Radiation
Fallout from nuclear weapons is estimated to be less than 1 millirem (MR) per year.
Watching TV provides a dose of about 1 millirem (mR) per year.
Porcelain false teeth or crowns yield about 0.1 millirem (mR) per year.
While it is true that there is a slight increase in radiation from living near a nuclear power plant, the average dose from living near a coal-fired power plant is typically on the order of 0.01 millirem (Mr) per year. Three times higher! This is due to the release of naturally occurring uranium/etc in the coal.
A plutonium-powered pacemaker delivers a dose rate of 100 millirems (mR) per year.
The Adequate Shielding Level
The Ionizing Radiation Regulations 1985 set an annual dose limit for radiation workers of 50 mSv/year. Any worker who exceeded 3/10 of this limit was designated as a "classified" radiation worker.
ie, 50 mSv/yr = 1 mSv/week = 25 µSv/hr (for a 40 hour working week)
3/10 of this limit = 25 x 3/10 = 7.5 µSv/hour = the adequate shielding level
However, the 1999 annual dose limit for ionizing radiation was lowered from 50 mSv/year to 20 mSv/year, but the adequate shielding level of 7.5 µSv/hour was maintained. Following the above formula with 20 mSv as an annual dose limit is meaningless as it reduces the adequate shielding level to 3 µSv/hour.
As this figure can be easily obtained at the University of Glasgow, this limit is fixed on all operating systems at entrances to "controlled" radiation areas.
In translating the above, we can surmise that radiation levels above 3 µSv/hour are treated as hazardous.
Radiation in Food
Foods naturally contain radioactive carbon-14, as well as small amounts of radioactive potassium. This gives an average dose of 20 millirem (mR) per year. In addition, some plants and animals naturally accumulate radioactive material, which is higher than the background dose rate.
The Human Body
The human body naturally contains Potassium, Carbon-14, and other radio nuclides. This makes it radioactive, to the tune of around 40 millirem (mR) a year. People are also radioactive, so a person could get slight doses from being around other people as well.
Radiation from X-Rays and Medical Tests
According to the American Nuclear Society, the following are the typical dose levels from various medical tests:
- Extremity (arm, leg, etc) Xray: 1 millirem (mR)
- Dental Xray: 1 millirem (mR)
- Chest Xray: 6 millirem (mR)
- Nuclear Medicine (thyroid scan): 14 millirem (mR)
- Neck/Skull Xray: 20 millirem (mR)
- Pelvis/Huip Xray: 65 millirem (mR)
- CAT Scan: 110 millirem (mR)
- Upper GI Xray: 245 millirem (mR)
- Barium Enema: 405 millirem (mR)
Totally all the expected exposure comes to around 300 millirem (mR) a year. The major source of the radiation dose rate is due to natural sources, radon, cosmic radiation, and terrestrial radiation. Man made sources of radiation are completely swamped by these natural sources in most cases.
The average total dose rate for the USA is 360 mrem a year. It has been estimated that your chance of dying from cancer increases 10% if you accumulate a total of 250,000 mrem. This would be over 3,000 mrem a year over 80 years, for example. This estimates presumably assume a linear risk factor between dose and the chance of getting cancer, and there are those who now dispute such assumptions, which means the risks from low levels of radiation may be overstated.
A single dose of around 450 R (450,000 mR) is usually considered produce death in 50% of the cases.

Information
What is radioactivity?
Radioactive decay, also known as nuclear decay or radioactivity, is the process by which a nucleus of an unstable atom loses energy by emitting particles of ionizing radiation. A substance that spontaneously emits such radiation, which includes the emission of energetic alpha particles, beta particles, and gamma rays, is considered radioactive.
There are several types of radioactive decay. A decay or loss of energy occurs when an atom with one type of nucleus, called the parent radionuclide, changes into an atom with a nucleus in a different state, or another nucleus with a different number of protons and neutrons. One of these products is called a daughter nuclide. In some decays the parent and daughter are different chemical elements, so the decay process causes a nuclear transformation (creating an atom of a new element).
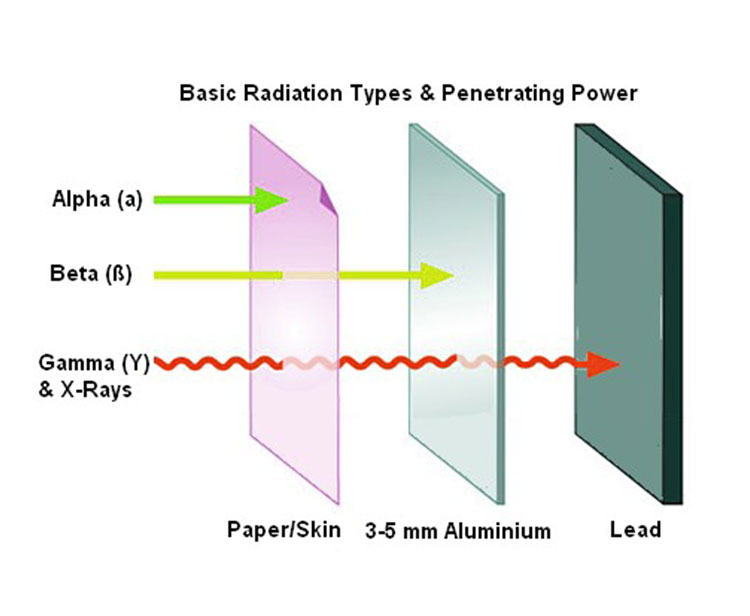
The first decay processes discovered were alpha decay, beta decay, and gamma decay. Alpha decay occurs when the nucleus emits an alpha particle (helium nucleus). This is the most common process in which nucleons are emitted, but in rarer types of decay, nuclei can eject protons or specific nuclei of other elements (a process called cluster decay). Beta decay occurs when the nucleus emits an electron or positron and a type of neutrino in the process of converting a proton into a neutron. The nucleus can capture an orbiting electron, converting a proton into a neutron (electron capture). All these processes result in nuclear transformation.
IONIZING RADIATION:
The first decay processes discovered were alpha decay, beta decay, and gamma decay. Alpha decay occurs when the nucleus emits an alpha particle (helium nucleus). This is the most common process in which nucleons are emitted, but in rarer types of decay, nuclei can eject protons or specific nuclei of other elements (a process called cluster decay). Beta decay occurs when the nucleus emits an electron or positron and a type of neutrino in the process of converting a proton into a neutron. The nucleus can capture an orbiting electron, converting a proton into a neutron (electron capture). All these processes result in nuclear transformation.

Information
Radioactive Elements
It is a list or list of radioactive elements. All elements have radioactive isotopes. This list contains elements that do not have stable isotopes. Each element is followed by the most stable isotope known and its half-life.
Click to view radioactive elements.
Read More
Information
How is radioactivity measured?
There are four different but interrelated units for measuring radioactivity, exposure, absorbed dose, and dose equivalent. These can be remembered by the mnemonic R-E-A-D, as follows, with both common (British, e.g., Ci) and international (metric, e.g., Bq) units in use:
Radioactivity refers to the amount of ionizing radiation released by a material. Whether it emits alpha or beta particles, gamma rays, x-rays, or neutrons, a quantity of radioactive material is expressed in terms of its radioactivity (or simply its activity), which represents how many atoms in the material decay in a given time period. The units of measure for radioactivity are the curie (Ci) and becquerel (BP).
Exposure describes the amount of radiation traveling through the air. Many radiation monitors measure exposure. The units for exposure are the roentgen (R) and coulomb/kilogram (C/kg).
Absorbed dose describes the amount of radiation absorbed by an object or person (that is, the amount of energy that radioactive sources deposit in materials through which they pass). The units for absorbed dose are the radiation absorbed dose (rad) and gray (Gy).
Dose equivalent (or effective dose) combines the amount of radiation absorbed and the medical effects of that type of radiation. For beta and gamma radiation, the dose equivalent is the same as the absorbed dose. By contrast, the dose equivalent is larger than the absorbed dose for alpha and neutron radiation, because these types of radiation are more damaging to the human body. Units for dose equivalent are the roentgen equivalent man (rem) and sievert (Sv), and biological dose equivalents are commonly measured in 1/1000th of a rem (known as a millirem or mrem mR).
For practical purposes, 1 R (exposure) = 1 rad (absorbed dose) = 1 rem or 1000 mrem (dose equivalent).
People are exposed on average to around 2 mSv of radiation a year from the natural environment, although there is considerable variation in this dose between individuals. The single-year limit for occupational exposure of workers is 50 mSv.
For the purposes of checking radioactivity levels on scrap, beta and gamma radiation is measured using a wide range of radioactivity meters, Geiger counters and Spectrometers. Reading is measured and recorded in terms of Microsieverts per Hour (uSv/hr) for the purposes of issuing Pre- Shipment Inspection Certificates.
Conversions or millirem (mR) To Millisieverts per Hour (mSv/hr) and Microsieverts per Hour (uSv/hr)
mR/hr |
mSv/hr |
uSv/hr |
Pre-shipment Inspection Services
At our company, we pride ourselves on the qualifications, training and motivation of our inspectors. Our team of experts undergo rigorous training programs to ensure they have the necessary skills and knowledge to perform inspections accurately and efficiently

Procedure for Inspections
Any Exporter / Importer who is seeking a Pre- Shipment Inspection Certificate
Read More
Inspectors and Team
At our company, we pride ourselves on the qualifications, training and motivation
Read More
Approved Territories
United Arab Emirates, Bahrain, Kuwait, Singapore, Malaysia, Hong Kong, etc...
Read More
Chartered Engineer's (CE) Certification
A Chartered Engineer’s (CE) Certification is required to export old or used Plants and Machinery to India.
Read More
Information About
RADIATION AND RADIOACTIVITY
Radiation is a process in which energetic particles or energetic waves travel through a vacuum, or through matter-containing media that are not required for their propagation.

